AAV-based gene therapies have demonstrated promising results in clinical trials, offering hope for treating a range of genetic disorders with a high degree of precision and efficacy.
The presence of empty and partially filled AAV particles in gene therapy formulations may limit the overall efficacy of the treatment by reducing the effective dosage of therapeutic genetic material delivered to target cells and potentially triggering an immune response. Therefore, detecting and quantifying these is vital for product efficacy and safety. Our services portfolio offers a range of analytical solutions ranging from visualization of the particles to obtaining quantitative data for objective evaluation of the specimen.
Visualize with CryoTEM
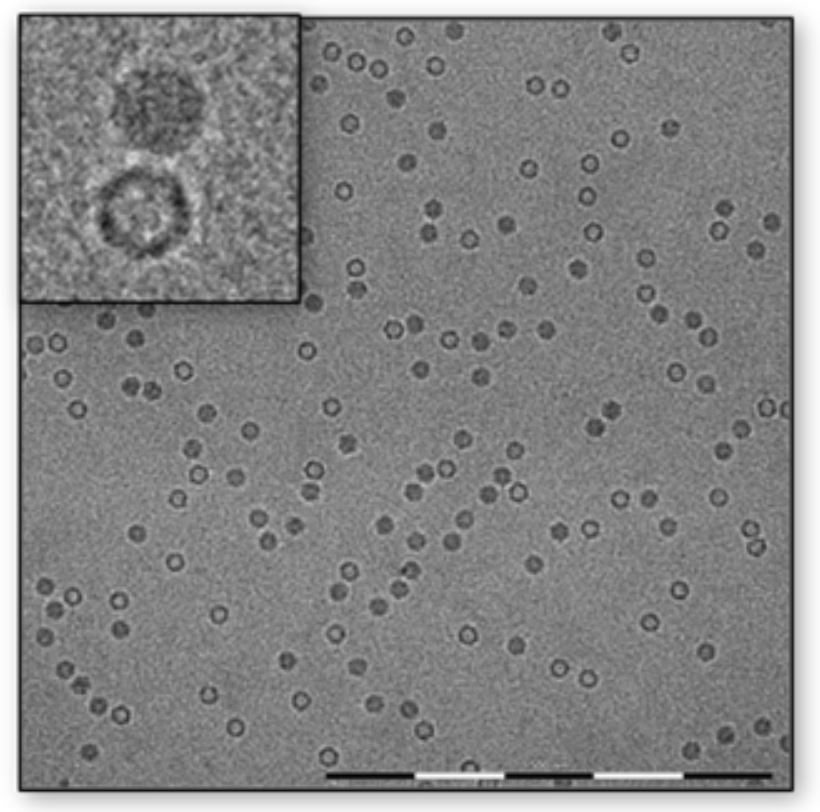
Our Cryogenic TEM (cryoTEM) method allows close-to-native inspection of the sample, from identifying AAV particles present in the specimen for obtaining general morphology attributes of the specimen, to distinguishing filled from empty capsids, as well as qualitatively evaluating the purity of the specimen and its integrity by identifying broken particles and debris present in the specimen.
Statistical Precision Through Image Analysis
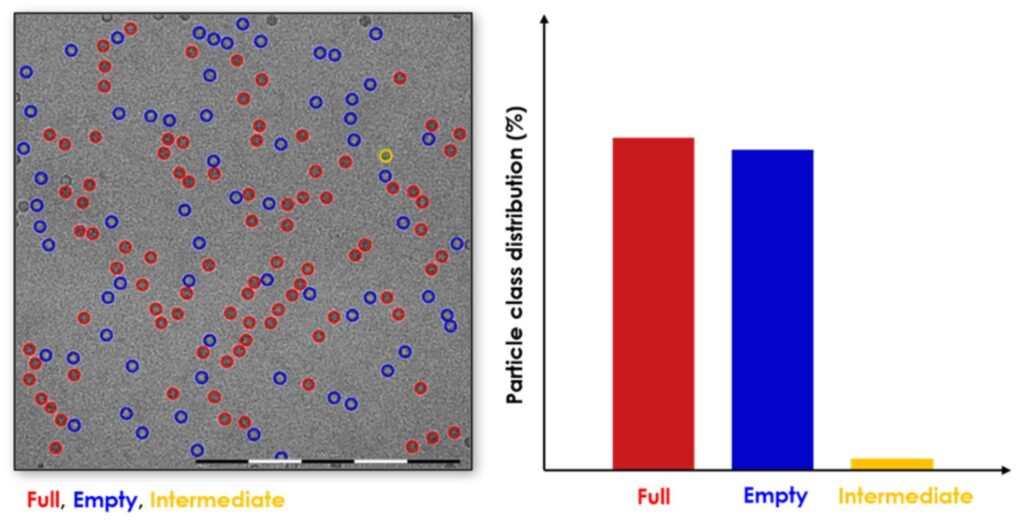
AAV particle packaging can be assessed using our proprietary image analysis software.
The automated workflows allow for a repeatable and reliable processing of datasets large enough for obtaining accurate statistical data from cryoTEM images.
Genome Length Determination
Our newly developed method for precise genome length determination provides quick insights into the efficiency of transgene incorporation.
Aggregation Insights
Beyond capsids, TEM reveals other impurities like host cell proteins and contaminants. Negative Stain TEM (nsTEM) provides details on overall morphology, integrity, and aggregation.
Aggregation, a challenge during purification, can be studied using nsTEM as an orthogonal technique.
Monitoring Product Quality Throughout the Entire Manufacturing Process
Both cryoTEM and nsTEM aid in monitoring and screening the product quality throughout the entire manufacturing process, and guiding decisions for product purification and safety.
Validation and Feasibility Studies
QuTEM provides method validation studies for AAV particles using both cryoTEM and nsTEM. Validate the method for your specific product with tests for specificity, accuracy, repeatability, and more following regulatory guidelines (e.g., ICH).